Histidine
Histidine, also referred to as L-histidine, is amino acid that was discovered back in 1896 by Albrecht Kossel and Sven Hedin - simultaneously but independently. The first researcher obtained Histidine by precipitation with mercuric chloride from the alkaline solution which contained the products of hydrolysis of the protamine – sturine. The second one isolated Histidine from the precipitate obtained by the base fraction from hydrolyzed protein being treated with silver nitrate and later with ammonia till the moment when a maximum precipitation occurred.
Chemical Structure of L-Histidine
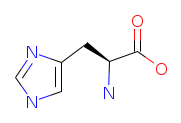
Identifiers and properties of Histidine
IUPAC Name: (2S)-2-Amino-3-(3H-imidazol-4-yl)propanoic acid
Symbol: Three-letter code - His. One-letter code - H
Molecular Weight (Molar Mass): 155.15456 g/mol
Molecular Formula (Structural Formula): C6H9N3O2
Canonical SMILES: C1=C(NC=N1)CC(C(=O)O)N
Isomeric SMILES: C1=C(NC=N1)C[C@@H](C(=O)O)N
InChIKey Identifier: HNDVDQJCIGZPNO-IFJFHIPQDC
CAS Number: 71-00-1
MDL Number: MFCD00064315
Melting point: 285 °C
RNA codons: CAU, CAC
Solubility in water: 41.6 g/1 L (25 °C); pKa - 1,82; pKb - 9,17
Rf value in n-butanol/acetic acid/water = 12:3:5 - 0.11
2D Molfile: Get the molfile
3D PDB file: Get the PDB file
Other names: (S)-4-(2-Amino-2-carboxyethyl)imidazole ; (S)-alpha-Amino-1H-imidazole-4-propanoic acid; L-2-Amino-3-(4-imidazolyl)propionic acid; (2S)-2-azaniumyl-3-(3H-imidazol-4-yl)propanoate
What are the functions of the Histidine?
Histidine is usually referred to as a semi-essential amino acid because it is nonessential in adults but is essential in the diet of infants and individuals with uremia - a kidney disorder.
Our body mostly needs Histidine to regulate and to utilize essential trace elements like iron, copper, molybdenum, zinc, and manganese. This amino acid is also essential in forming numerous metal-bearing enzymes and compounds, such as the antioxidant super oxide dismutase.
Meanwhile, many toxic metals like mercury, lead, and cadmium, along with threatening excess of essential minerals like zinc and copper, usually stimulate the rapid formation of metallothionein inside the cells of your brain, liver, and kidneys to protect these cells. Metallothionein needs Histidine to be formed, so people without a Histidine diet whose body is contaminated with heavy metals are subject to the body's depletion of adequate stores of this amino acid, thus causing mineral-enzyme deficiencies and dysregulations. This body condition is best recognized by dysfunction of Histidine-dependent compounds, coupled with low blood plasma concentration of it.
By the way, if you do not tolerate sulfur-bearing food, such as broccoli and garlic, and show the elevated blood plasma L-cysteine levels, it means that you suffer from inactivation of Histidine and of iron-dependent enzyme cysteine dioxygenase.
Food sources
Meat and Poultry. Chicken Breast: A 3-ounce (85-gram) serving of roasted chicken breast provides approximately 0.6 grams of histidine. Beef: A 3-ounce (85-gram) serving of cooked beef contains around 0.5 grams of histidine.
Fish. Tuna: A 3-ounce (85-gram) serving of cooked tuna provides about 0.6 grams of histidine. Salmon: Similar to tuna, salmon contains around 0.6 grams of histidine per 3-ounce (85-gram) serving.
Dairy Products. Milk: One cup of milk contains about 0.1 grams of histidine. Cheese: Different types of cheese provide varying amounts of histidine, typically ranging from 0.1 to 0.5 grams per ounce.
Eggs. One large egg provides approximately 0.1 grams of histidine.
Legumes. Soybeans: A 1-cup serving of cooked soybeans contains around 0.6 grams of histidine. Lentils: A 1-cup serving of cooked lentils provides about 0.2 grams of histidine.
Nuts and Seeds. Sunflower Seeds: A 1-ounce (28-gram) serving of sunflower seeds contains approximately 0.2 grams of histidine. Pumpkin Seeds: Pumpkin seeds provide around 0.1 grams of histidine per ounce.
Whole Grains. Quinoa: A 1-cup serving of cooked quinoa provides about 0.3 grams of histidine. Brown Rice: A 1-cup serving of cooked brown rice contains approximately 0.1 grams of histidine.
Seafood. Shrimp: A 3-ounce (85-gram) serving of cooked shrimp provides around 0.4 grams of histidine. Crab: Crab is another seafood source with approximately 0.4 grams of histidine per 3-ounce (85-gram) serving.
Dense Protein Sources. Whey Protein: One scoop (around 30 grams) of whey protein powder can provide 0.5-1 gram of histidine.
Cabbage Family Vegetables. Spinach: A 1-cup serving of cooked spinach contains about 0.1 grams of histidine.
These values are approximate and can vary based on factors such as cooking methods, specific varieties of food, and preparation techniques. If you have specific health concerns or conditions that may require additional histidine, it's advisable to consult with a healthcare professional or a registered dietitian for personalized advice.