Amino Acids
Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger RNA (mRNA) translation.
There are over 700 types of amino acids that have been discovered in nature. Almost all of them are α-amino acids. They have been found in:
• bacteria
• fungi
• algae
• plants.
Amino acids can best be described as the construction blocks from which protein is made. Just as in a child's construction kit the pieces come in different shapes and sizes and yet fit together to make something recognizable, so the more than 20 amino acids each have unique characteristics, and yet are capable of being fitted together into an almost limitless variety of proteins.
Protein is formed by the joining together, into chains, of amino acids and thus far over 100,000 different proteins have been identified in nature, which are the result of variations in the pattern in which the chains are constructed.
The human body alone contains over 50,000 different forms of protein. The total presence of amino acids in the body represents fully three quarters of the body's dry weight (this is excluding the water content). Most of the amino acids in the body can be manufactured out of just eight other amino acids, which are all essential in the diet. This means that our diet has to allow the acquisition of free forms of these eight amino acids for life to continue.
These 'essential' amino acids are critically important to life and health, for out of them the body makes the other amino acids, as well as many of the vital compounds which keep the body working, such as the enzymes, neurotransmitters, mucopolysaccharides etc., not to mention blood, muscles, organs and bones from which we are constructed.
When only a short chain of amino acids is joined together, in a particular sequence, it is called a peptide. When the chain is long, it is called a protein.
The amino acids themselves are constructed from a combination of the following elements: carbon, hydrogen, oxygen, nitrogen and in some cases sulphur.
L- and D-amino acids
Every amino acid comes in two forms, a 'left-handed' (L) and a 'right-handed' (D) form. These two forms are identical in every respect except for the conformation of the subunits of which they are composed. That is to say, although chemically they contain the same elements, in precisely the same quantities and in the same sequence, they are the mirror image of each other, just as the human left hand has the same construction as the human right hand and yet they are different (a right hand cannot wear a left-handed glove for example). Protein chains cannot be formed from a combination of L and D amino acids.
The body is constructed almost without exception from the L forms of amino acid. However, the D forms, which occur in nature, are often found to have therapeutic value, for example, the D form of phenylalanine is a particularly valuable asset in treating pain.
Abbreviations
To understand the amino acids' abbreviation, it is important to know why their names have been shorten in the first place. A reason is to make them easy to identify and to use more manageable three-letter system. For instance, the simplest amino acid, glycine is depicted as H—Gly—OH, with the «H» and the «OH» being «H2O», which represents the H2O at the time of amino acid condensing in order to form a peptide.

Another way to look at the three-letter abbreviation system is that it captures the amino acid residual state, which comprises proteins and peptides. When the system was introduced, it was thought primarily to save space, rather than simplify amino acid names. It is important to know that, when one-letter system is used, such as «G» for glycine, which is more commonly used nowadays, it is referring to synthesized peptides from the coded amino acids groups.
How Amino Acids were Discovered
The amino acids are a result of protein hydrolysis. Throughout the centuries, amino acids have been discovered in a variety of ways, though primarily by way of chemists and biochemists of high intelligence who possessed the greatest skills and patience and who were innovative and creative in their work.
Protein chemistry is age-old, with some dating back thousands of years ago. Processes and technical applications such as glue preparation, cheese manufacturing and even the discovery of ammonia via the filtering of dung, occurred centuries ago. Moving forward in time to 1820, Braconnot prepared glycine directly from gelatin. He was attempting to uncover whether proteins acted like starch or whether they are made of acids and sugar.
While progress was slow at that time, it has since gained plenty of speed, although the complicated processes of protein composition have not entirely been uncovered even to this day. But many years have gone by since Braconnot first initiated such observations.
Much more should be discovered in the analysis of amino acids as well as finding new amino acids. The future of protein and amino acids chemistry is lying in biochemistry. Once that is accomplished—but only until then will our knowledge of amino acids and proteins be satiated. Yet it is likely that day will not come anytime soon. This all adds to the mystery, complexities and strong scientific value of amino acids.
What foods contain amino acids?
Essential amino acids can be obtained by maintaining a protein-rich diet, available in various plant and animal foods.
Complete proteins, containing all 20 or more types of amino acids, are present in certain foods. Examples include red meat, chicken, fish, seafood, eggs, milk, cheese, yogurt, quinoa, chia seeds, and tofu. These foods are comprehensive sources of essential amino acids.
Conversely, some foods are incomplete proteins, lacking one or more of the nine essential amino acids. Plant-based sources like grains, nuts, seeds, beans, legumes, fruits, and vegetables fall into this category. If you follow a vegetarian or vegan diet, incorporating a variety of these incomplete proteins is essential to ensure the intake of all necessary amino acids.
How many amino acids does your body need?
While it's not necessary to consume foods rich in amino acids with every meal, maintaining a balanced intake throughout the day is crucial. The recommended daily allowance for each of the essential amino acids is based on body weight, with the suggested amount provided for every 2.2 lbs:
- Histidine: 14 milligrams
- Isoleucine: 19 milligrams
- Leucine: 42 milligrams
- Lysine: 38 milligrams
- Methionine: 19 milligrams
- Phenylalanine: 33 milligrams
- Threonine: 20 milligrams
- Tryptophan: 5 milligrams
- Valine: 24 milligrams
Benefits of amino acids
Protein Synthesis. Amino acids are the building blocks of proteins. They are essential for the synthesis of structural proteins
Muscle Growth and Repair. Branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine are particularly important for muscle protein synthesis, aiding in muscle growth and repair.
Neurotransmitter Production. Amino acids like tryptophan and tyrosine are precursors to neurotransmitters serotonin and dopamine, respectively, influencing mood, cognition, and behavior.
Energy Production. Certain amino acids can be converted into energy, especially during periods of increased physical activity or low carbohydrate availability.
Immune Function. Amino acids contribute to the production of antibodies and immune system components, playing a role in immune function and response.
Hormone Regulation. Amino acids are involved in the synthesis of hormones such as insulin, growth hormone, and thyroid hormones, contributing to metabolic regulation.
Wound Healing. Amino acids, especially arginine and glutamine, are involved in the wound healing process and tissue repair.
Collagen Formation. Proline and lysine are crucial for the synthesis of collagen, a structural protein that supports skin, bones, and connective tissues.
Classifications
Experts classify amino acids based on a variety of features, including whether people can acquire them through diet. Accordingly, scientists recognize three amino acid types:
1. Nonessential
2. Essential
3. Conditionally essential
However, the classification as essential or nonessential does not actually reflect their importance as all 20 amino acids are necessary for human health.
Eight of these amino acids are essential (or indispensable) and cannot be produced by the body. They are:
• Leucine
• Isoleucine
• Lysine
• Threonine
• Methionine
• Phenylalanine
• Valine
• Tryptophan
Histidine is an amino acid that is categorized as semi-essential since the human body doesn't always need it to properly function therefore dietary sources of it are not always essential. Meanwhile, conditionally essential amino acids aren't usually required in the human diet, but do become essential under certain circumstances.
Finally, nonessential amino acids are produced by the human body either from essential amino acids or from normal protein breakdowns. Nonessential amino acids include:
• Asparagine
• Alanine
• Arginine
• Aspartic acid
• Cysteine
• Glutamic acid
• Glutamine
• Proline
• Glycine
• Tyrosine
• Serine
An additional amino acids' classification depends upon the side chain structure, and experts recognize these five as:
• Cysteine and Methionine (amino acids containing sulfur)
• Asparagine, Serine, Threonine, and Glutamine (neutral amino acids)
• Glutamic acid and Aspartic acid (acidic); and Arginine and Lysine (basic)
• Leucine, Isoleucine, Glycine, Valine, and Alanine (aliphatic amino acids)
• Phenylalanine, Tryptophan, Tyrosine and Histidine (aromatic amino acids)
One final amino acid classification is categorized by the side chain structure that divides the list of 20 amino acids into four groups - two of which are the main groups and two that are subgroups. They are:
1. Non-polar
2. Polar
3. Acidic and polar
4. Basic and polar
For example, side chains having pure hydrocarbon alkyl or aromatic groups are considered non-polar, and these amino acids are comprised of Phenylalanine, Glycine, Valine, Leucine, Alanine, Isoleucine, Proline, Methionine and Tryptophan. Meanwhile, if the side chain contains different polar groups like amides, acids and alcohols, they are classified as polar. It includes Tyrosine, Serine, Asparagine, Threonine, Glutamine, and Cysteine. If the side chain contains carboxylic acid, the amino acids in the acidic-polar classification are Aspartic Acid and Glutamic Acid. Furthermore, if the side chain consists of a carboxylic acid and basic-polar, these amino acids are Lysine, Arginine, and Histidine.
Properties of Amino Acids (pKa, pKb, pKx, pl)
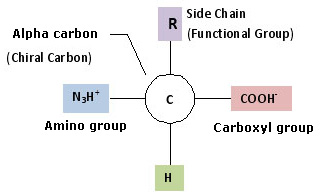
The properties of α-amino acids are complex, yet simplistic in that every molecule of an amino acid involves two functional groups: carboxyl (-COOH) and amino (-NH2).
Each molecule can contain a side chain or R group, e.g. Alanine is an example of standard amino acid containing methyl side chain group. The R groups have a variety of shapes, sizes, charges, and reactivities. This allows amino acids to be grouped according to the chemical properties of their side chains.
Table of common amino acid abbreviations and properties
| Name | Three letter code | One letter code | Molecular Weight |
Molecular Formula |
Residue Formula |
Residue Weight (-H2O) |
pKa | pKb | pKx | pl |
| Alanine | Ala | A | 89.10 | C3H7NO2 | C3H5NO | 71.08 | 2.34 | 9.69 | – | 6.00 |
| Arginine | Arg | R | 174.20 | C6H14N4O2 | C6H12N4O | 156.19 | 2.17 | 9.04 | 12.48 | 10.76 |
| Asparagine | Asn | N | 132.12 | C4H8N2O3 | C4H6N2O2 | 114.11 | 2.02 | 8.80 | – | 5.41 |
| Aspartic acid | Asp | D | 133.11 | C4H7NO4 | C4H5NO3 | 115.09 | 1.88 | 9.60 | 3.65 | 2.77 |
| Cysteine | Cys | C | 121.16 | C3H7NO2S | C3H5NOS | 103.15 | 1.96 | 10.28 | 8.18 | 5.07 |
| Glutamic acid | Glu | E | 147.13 | C5H9NO4 | C5H7NO3 | 129.12 | 2.19 | 9.67 | 4.25 | 3.22 |
| Glutamine | Gln | Q | 146.15 | C5H10N2O3 | C5H8N2O2 | 128.13 | 2.17 | 9.13 | – | 5.65 |
| Glycine | Gly | G | 75.07 | C2H5NO2 | C2H3NO | 57.05 | 2.34 | 9.60 | – | 5.97 |
| Histidine | His | H | 155.16 | C6H9N3O2 | C6H7N3O | 137.14 | 1.82 | 9.17 | 6.00 | 7.59 |
| Hydroxyproline | Hyp | O | 131.13 | C5H9NO3 | C5H7NO2 | 113.11 | 1.82 | 9.65 | – | – |
| Isoleucine | Ile | I | 131.18 | C6H13NO2 | C6H11NO | 113.16 | 2.36 | 9.60 | – | 6.02 |
| Leucine | Leu | L | 131.18 | C6H13NO2 | C6H11NO | 113.16 | 2.36 | 9.60 | – | 5.98 |
| Lysine | Lys |
K | 146.19 | C6H14N2O2 | C6H12N2O | 128.18 | 2.18 | 8.95 | 10.53 | 9.74 |
| Methionine | Met | M | 149.21 | C5H11NO2S | C5H9NOS | 131.20 | 2.28 | 9.21 | – | 5.74 |
| Phenylalanine | Phe | F | 165.19 | C9H11NO2 | C9H9NO | 147.18 | 1.83 | 9.13 | – | 5.48 |
| Proline | Pro | P | 115.13 | C5H9NO2 | C5H7NO | 97.12 | 1.99 | 10.60 | – | 6.30 |
| Pyroglutamatic | Glp | U | 139.11 | C5H7NO3 | C5H5NO2 | 121.09 | – | – | – | 5.68 |
| Serine | Ser | S | 105.09 | C3H7NO3 | C3H5NO2 | 87.08 | 2.21 | 9.15 | – | 5.68 |
| Threonine | Thr | T | 119.12 | C4H9NO3 | C4H7NO2 | 101.11 | 2.09 | 9.10 | – | 5.60 |
| Tryptophan | Trp | W | 204.23 | C11H12N2O2 | C11H10N2O | 186.22 | 2.83 | 9.39 |
– | 5.89 |
| Tyrosine | Tyr | Y | 181.19 | C9H11NO3 | C9H9NO2 | 163.18 | 2.20 | 9.11 | 10.07 | 5.66 |
| Valine | Val | V | 117.15 | C5H11NO2 | C5H9NO | 99.13 | 2.32 | 9.62 | – | 5.96 |
Amino acids are crystalline solids which usually are water soluble and only sparingly dissoluble in organic solvents. Their solubility depends on the size and nature of the side chain. Amino acids have very high melting points, up to 200-300°C. Their other properties varying for each particular amino acid.
Isoelectric points of amino acids
The isoelectric point (pI) of an amino acid refers to the pH at which the amino acid exists in its neutral, or zwitterionic, form. At the isoelectric point, the amino acid carries no net electrical charge because the positive charge on the amino group (+NH3) equals the negative charge on the carboxyl group (−COO−). The isoelectric point is an essential concept in biochemistry and plays a role in various biological processes, including protein behavior and separation techniques.
pI calculation. The pI is the pH at which an amino acid is electrically neutral. For amino acids with ionizable side chains, determining the pI involves considering the ionization of the amino and carboxyl groups, as well as the side chain (R-group). For example, the pI of the amino acid glycine is around 5.97, while the pI of lysine, with a basic side chain, is approximately 9.74.
The isoelectric point is crucial in the study of proteins and their behavior in different environments. Proteins, being composed of amino acids, exhibit characteristics related to their charge at different pH levels. Techniques like isoelectric focusing use the principles of the isoelectric point to separate proteins based on their pI values in an electric field.
Zwitterionic Form of amino acids
Since the amino and the acidic groups have opposite electrical charges, an AA (an amphoteric electrolyte) acts as a base or an acid by accepting and supplying a hydrogen ion, respectively. Thus, all AA form intramolecular salts both in the crystalline state and in aqueous solution. This structure, in which a molecule has both positive and negative electrical charges, is known as a dipolar ion or zwitterion (ionizable). Uncharged (nondissociated) forms of AA almost do not exit.
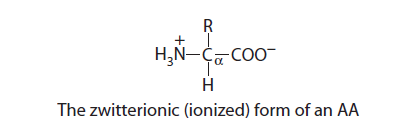
For example, the ratio of charged dipolar (ionized α-amino and α-carboxyl groups) to uncharged forms of l-aspartic acid in aqueous solution is approximately 28,000:1 at pH 7.0. Similarly, the proportion of charged dipolar (ionized α-amino and α-carboxyl groups) to uncharged forms of l-lysine in aqueous solution is approximately 320,000:1 at pH 7.0.
Amino acids and neurotransmitters
Individual types of amino acids have particular character-istics. Some are capable of influencing body processes because they are essential to the formation of neurotrans-mitters, substances which are used in the brain and by the nervous system to increase or decrease the efficiency and rapidity of nerve transmission. The ability of the brain to receive and to transmit messages depends upon these neurotransmitters, which are themselves dependent upon particular amino acids. All functions of the body depend upon sound nervous interconnection. This allows organs and muscles to report back to the higher centres as to their status, and for receiving instructions from the higher centres, as to their behaviour and needs. The coordination and regulation of all the millions of messages that are constantly going on in the body, depend upon neurotransmitters and therefore on amino acids. Amino acids are especially important where nerves interact (synapse), where information is passed on and received. Some of the neurotransmitters have a stimulating, excitatory function and others have a calming, inhibitory function.
The scope and use of appropriate amino acids in therapy can therefore be seen to be enormous. Unless all the amino acids, in their free form, are present in adequate amounts, there will be imbalances in the neurotransmitter function, and a variety of nervous and emotional problems will result. The very energy of the brain is dependent upon certain amino acids. The tryptophan and phenylalanine, are both of profound importance in their relation to brain and nerve function.
Another major area of activity of some of the amino acids is as detoxifiers of the body. The sulphur rich amino acids (methionine, cysteine, cystine) are especially capable of this sometimes life-saving task. These have the ability to chelate (lock onto) Heavy metals such as lead, mercury and aluminium, which are toxic to the body, and to actually remove them from the system.
They are also capable of damping down damaging processes in the body relating to oxidation of certain substances such as fats. When toxic substances are present in tissue or in the bloodstream, there is potential for what is called free radical damage, as fractions of the oxidizing substance cascade around the area creating tissue damage. These processes which are thought to result in such cell changes as occur in arteries before they become atherosclerotic, and to cells before they become cancerous, are controlled by free radical scavengers or quenchers, of which the sulphur rich amino acids are a major part. Vitamins A, C, and E and the mineral selenium are also anti-oxidants which reduce free radical damage.
20 Amino Acids and their Functions
Only 20 amino acids are found in the human peptides and proteins. These naturally occurring amino acids are used by cells to synthesize peptides and proteins. They are typically identified by generic formula: H2NCHRCOOH.
The primary difference between the 20 amino acids is a different structure of R group. Below the essential amino acids and their respective functions are shown.
Non-polar, aliphatic residues
 |
Glycine (G/Gly). Slices DNA and produces different amino acids. One of the three most important glycogenic amino acids. Read more about Glycine. |
 |
Alanine (A/Ala). Important source of energy for muscle. One of the three most important glycogenic amino acids. The primary amino acid in sugar metabolism. Boosts immune system by producing antibodies. Read more about Alanine. |
 |
Valine (V/Val). Essential for muscle development. Read more about Valine. |
 |
Leucine (L/Leu). Beneficial for skin, bone and tissue wound healing. Read more about Leucine. |
 |
Isoleucine (I/Ile). Necessary for the synthesis of hemoglobin. Read more about Isoleucine. |
 |
Proline (P/Pro). Critical component of cartilage, aids in joint health, tendons and ligaments. Keeps heart muscle strong. Read more about Proline. |
Aromatic residues
 |
Phenylalanine (F/Phe). Beneficial for healthy nervous system. It boosts memory and learning. Read more about Phenylalanine. |
 |
Tyrosine (Y/Tyr). Precursor of dopamine, norepinephrine and adrenaline. Increases energy, improves mental clarity and concentration, can treat some depressions. Read more about Tyrosine. |
 |
Tryptophan (W/Trp).Necessary for a synthesis of neurotransmitter serotonin. Effective sleep aid, due to conversion to serotonin. Reduces anxiety and some forms of depression. Treats migraine and headaches. Stimulates growth hormone Read more about Tryptophan. |
Polar, non-charged residues
 |
Serine (S/Ser). One of the three most important glycogenic amino acids, the others being alanine and glycine. Maintains blood sugar levels, and boosts immune system. Myelin sheaths contain serine. Read more about Serine. |
 |
Threonine (T/Thr). Required for formation of collagen. Helps prevent fatty deposits in liver. Aids in antibodies' production. Read more about Threonine. |
 |
Cysteine (C/Cys). Protective against radiation, pollution and ultra-violet light. Detoxifier, necessary for growth and repair of skin. Read more about Cysteine. |
 |
Methionine (M/Met). An antioxidant. Helps in breakdown of fats and aids in reducing muscle degeneration. Read more about Methionine. |
 |
Asparagine (N/Asn).One of the two main excitatory neurotransmitters. Read more about Asparagine. |
 |
Glutamine (Q/Gln). Essential for helping to maintain normal and steady blood sugar levels. Helps muscle strength and endurance. Gastrointestinal function, provides energy to small intestines. Read more about Glutamine. |
Positively charged residues
 |
Lysine (K/Lys). Component of muscle protein, needed in the synthesis of enzymes and hormones. It is also a precursor for L-carathine, which is essential for healthy nervous system function. Read more about Lysine. |
 |
Arginine (R/Arg). One of the two main excitatory neurotransmitters. May increase endurance and decrease fatigue. Detoxifies harmful chemicals. Involved in DNA synthesis. Read more about Arginine. |
 |
Histidine (H/His). Found in high concentrations in hemoglobin. Treats anemia, has been used to treat rheumatoid arthritis. Read more about Histidine. |
Negatively charged residues
 |
Aspartate (D/Asp). Increases stamina and helps protect the liver; DNA and RNA metabolism, immune system function. Read more about Aspartate. |
 |
Glutamate (E/Glu). Neurotransmitter that is involved in DNA synthesis. Read more about Glutamate. |
Amino acid catabolism
Amino acid catabolism, also known as amino acid degradation, is a fundamental process that involves the breakdown of amino acids into simpler molecules. This intricate biochemical pathway is essential for maintaining nitrogen balance, generating energy, and providing intermediates for various metabolic processes. For more detailed information about amino acid catabolism, you can refer to the diagram created by Mikael Häggström (via Wikimedia Commons).

Glucogenic amino acid
A glucogenic amino acid, also known as a glucoplastic amino acid, is an amino acid capable of undergoing conversion into glucose through the process of gluconeogenesis. This stands in contrast to ketogenic amino acids, which are transformed into ketone bodies.
The conversion of glucogenic amino acids into glucose follows a two-step process. Initially, these amino acids are transformed into alpha-keto acids. Subsequently, these alpha-keto acids undergo further conversion into glucose. Both of these crucial processes take place primarily in the liver. This mechanism becomes particularly prominent during catabolism, becoming more pronounced as the severity of fasting and starvation increases.
In humans, the glucogenic amino acids are: Alanine; Arginine; Asparagine; Aspartic acid; Cysteine; Glutamic acid; Glutamine; Glycine; Histidine; Methionine; Proline; Serine; Valine.
Ketogenic amino acid
A ketogenic amino acid is an amino acid capable of being directly degraded into acetyl-CoA. Acetyl-CoA serves as the precursor for ketone bodies and myelin, particularly crucial during early childhood when there is a heightened demand for myelin synthesis in the developing brain. Unlike glucogenic amino acids, ketogenic amino acids cannot be converted into glucose, as both carbon atoms in the resulting ketone bodies are ultimately metabolized into carbon dioxide through the citric acid cycle.
In humans, two amino acids, leucine and lysine, are exclusively ketogenic. Additionally, there are amino acids with dual characteristics, being both glucogenic and ketogenic, referred to as amphibolic. A helpful mnemonic for remembering these amino acids is "PITTT": phenylalanine, isoleucine, threonine, tryptophan, and tyrosine.
Conclusion
In considering amino acids in relation to health and ill health there are two main areas to cover. The first looks at particular conditions relating to disorders of amino acid metabolism, resulting in a related pathological state. The second area, and the one which attracts the major interest among nutritionally orientated practitioners, is that involving conditions not specifically related to diseases of amino acid metabolism, and yet which appear to respond positively to dietary manipulation which involves the intake of particular amino acids (and other nutrients).
Such conditions as certain forms of depression; insomnia; herpes infections; weight problems; fat metabolism dysfunction; epilepsy, etc. have all been shown to improve, in suitable cases, by the use of appropriate amino acid therapy. Certain physiological functions have also been enhanced by the selective use of amino acids. These include detoxification of heavy metals; modification of free radical activity; enhanced mental function via neurotransmitter stimulation etc.
The ability of the brain neurones to manufacture and utilize a number of neurotransmitters, such as serotonin, acetylcholine and, it is conjectured, the catecholamines, dopamine and norepinephrine, is dependent upon the concentrations of both the amino acids and choline in the bloodstream. This largely depends upon the food composition at the previous meal.2 Since the brain is apparently unable to make adequate quantities of amino acids and choline to meet its requirements for neurotransmitter synthesis it is vital that adequate quantities of these precursors are present in the circulation. In the current context it is pertinent to simply be aware of the vital role played by amino acids in brain function. It is pointed out that the dry material of the brain comprises more than one third protein, and that stress can create a situation in which non-essential amino acids cannot be adequately produced to meet its needs. A number of researchers have shown that such a situation can result in a range of mento-emotional symptoms, such as depression, apathy, irritability, etc. The subsequent imbalance in uric acid levels resulting from incomplete amino acid synthesis, and consequent utilization of free amino acids as fuel by the body, can result in self mutilating behaviour in children.